
Wenyuan Health
Your Local Partner in Singapore Healthcare
End-to-end support for supplements and cosmetics — from regulatory approval to manufacturing, sales, and market entry.
Our Core Services
Regulatory-first, market-driven

Registration & Regulatory
We support product registration and compliance for:
1.Health supplements
2.Traditional medicines
3.OTC pharmaceutical products
4.Cosmetic Products
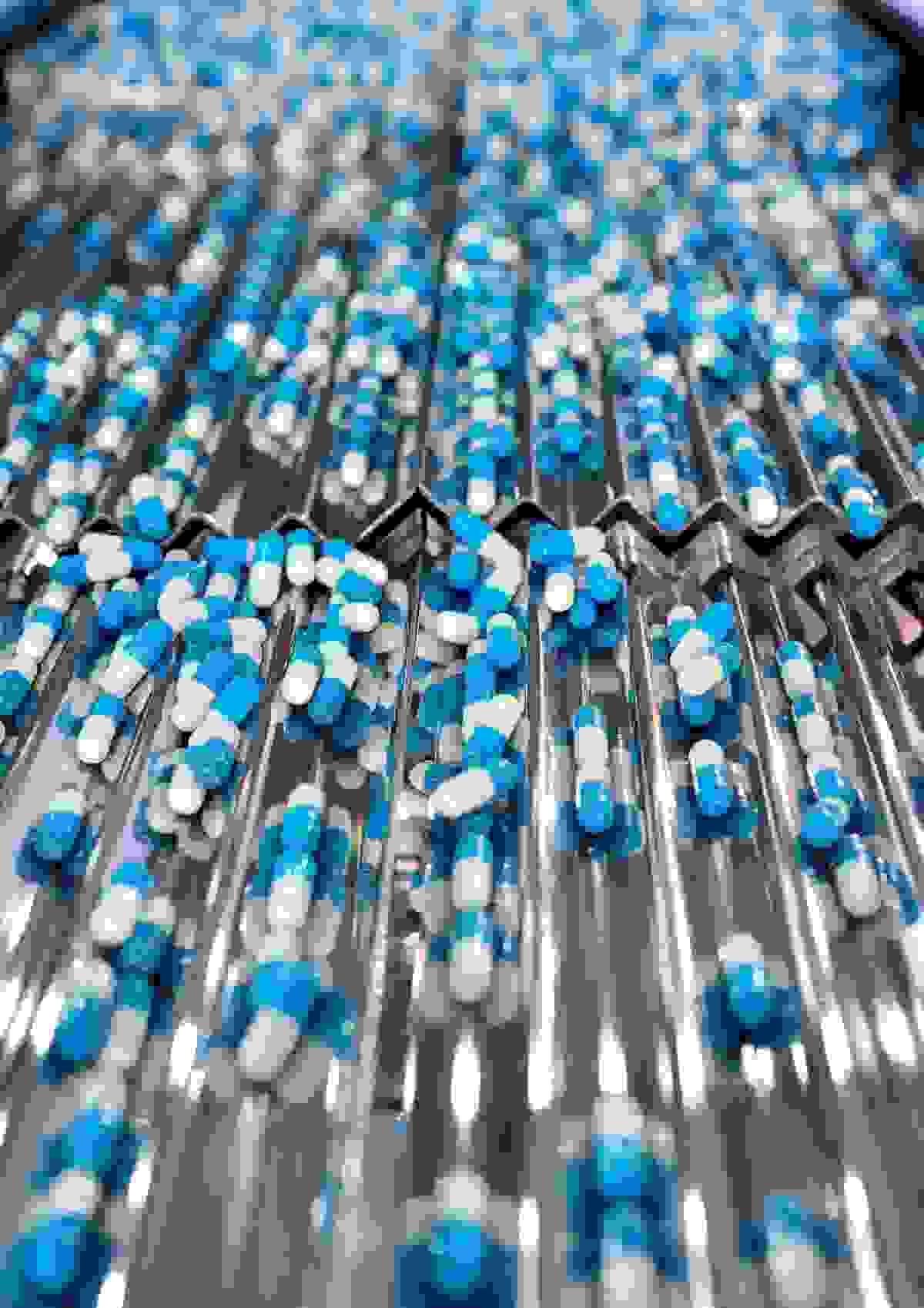
Manufacturing Service
We support local manufacturing and processing through qualified facilities in Singapore:
1.Contract manufacturing (OEM / ODM)
2.Local processing and packaging
3.Formulation and testing

Sales & Marketing
We provide practical, on-the-ground sales and marketing support, including:
1.Distribution channel development
2.Content, campaign, and launch event
From Formula to Finished Product



We support healthcare, supplement and cosmetic brands with local processing and compliance-ready production in Singapore.
From regulatory alignment to local processing, packaging, and production coordination, we help ensure products meet Singapore standards and are ready for market entry.
Who We Work With
Supplement and healthcare brands
Ingredient owners and formulation teams
Pharmaceutical-related companies
Start-up companies
OEM & ODM
Structured Service Packages

Regulatory Alignment
- Regulatory assessment & product classification
- Documentation preparation
- Labeling and compliance review
- Coordination with relevant authorities

Production & Quality Coordination
- Formulation Service
- Product design & Barcode application
- Local processing & repackaging
- Pilot runs and small-batch production

Product Qualification
Certificate of Analysis (COA)
Certificate of Origin (COO)
Free Sale Certificate (FSC / CFS)

Oversea Market
Import and export supporting documents
Trade and logistics-related paperwork coordination
Overseas Product Registration Documents
How We Work

Step 1 – Requirement Briefing
Product, market, and compliance requirements are clearly defined.

Step 2 – Project Discussion
Scope, timeline, and processing approach are aligned.

Step 3 – Quotation & Agreement
A formal quotation is provided, followed by contract confirmation.
Step 4 – Sample or Production Processing
Sample processing or commercial production coordination in Singapore.
Step 5 – Testing & Quality Review
Testing, documentation, and acceptance prior to release.

Step 6 – Market Entry Support
Product distribution and marketing coordination to support market entry.

About Us
EMBRACING HEALTH
Mission: Focus on healthcare industry and make business easier to sustain and grow.
Vision: Continue to level up the business standard together.
Trusted by Our Partners
#Review 1
Early-Stage Supplement Brand (Singapore)
"As a startup, we needed clear guidance on what was realistically feasible in SG. Wenyuan Health supported us from formulation planning through processing and compliance, helping us avoid unnecessary detours early on."
— Founder
#Review 2
Regional Healthcare Distributor
"We engaged Wenyuan Health for local processing and secondary packaging in Singapore. The coordination was efficient, documentation was well aligned, and communication remained clear throughout the process.
— Operations Manager
"Wenyuan Health supported the regulatory preparation for more than 20 products. The team worked in a structured and consistent manner, which made multi-SKU coordination much easier."
— Regulatory & Market Lead
#Review 3
Chain Medical Clinics
"Setting up a GMP facility in Singapore involved many regulatory and operational layers.
Wenyuan Health provided practical support across planning, authority coordination, and quality system alignment."
— Overseas Business Director
#Review 4
China Pharmaceutical Company
Frequently Asked Questions
Are you a manufacturer or a processing partner?
We are a processing and execution partner.
We coordinate with qualified local facilities and support compliance, documentation, and production readiness based on project needs.
Can you support both sample runs and commercial production?
Yes. We support sample preparation, pilot runs, and commercial-scale production coordination, depending on product type and project stage.
Do you assist with regulatory and product documentation?
Yes. We support documentation preparation and coordination, including product dossiers, COA, COO, Free Sale Certificate, and other regulatory or trade-related documents required for processing and market entry.
Which markets do you support besides Singapore?
Our core execution is based in Singapore.
We also support selected overseas and ASEAN market projects through local partners, subject to regulatory requirements.
How do you ensure compliance and quality alignment?
We work with established quality systems and align processing activities with applicable regulatory, GMP, and documentation requirements throughout the project.
How do we get started?
You can start by sharing your product and market requirements.
We will review feasibility and arrange a discussion to align on scope and next steps.
Get Started
Let’s Bring Your Product to Market — Compliantly and Locally
We support local processing, compliance preparation, and production coordination in Singapore — helping products move smoothly from documentation to market entry.
Selected Case Studies
Background A healthcare brand planned to enter an ASEAN market with a portfolio of more than 20...Background A healthcare brand planned to launch a probiotic powder sachet product targeting daily...What should a manufacturing health supplement company do to obtain its factory licence in...
© 2025 Wenyuan Health Pte. Ltd.
All rights reserved.



















